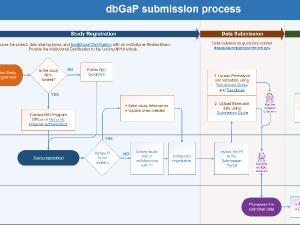
This page describes how to register and submit data from NIH-funded studies to dbGaP.
- Submitting data to dbGaP is not a requirement under the GDS policy, however, all human studies are registered in dbGaP even if they are not submitted to dbGaP.
- Some ICs or data sharing agreements require submission to specific repositories. However, the study must still be registered in dbGaP.
- Studies that have been granted an alternate data sharing plan may not have to submit data to dbGaP, but must still register the study in dbGaP.
Step 1: Identify appropriate Genomic Program Administrator (GPA)
Each funding institute or center (IC) has designated staff, referred to as GPAs, to assist investigators with registering and submitting genomic data. Note that an investigator cannot register a study in dbGaP by themselves.
-
For Extramural (Grants & Contracts) and NIH Intramural investigators:
- Consult with your Program Officer to identify the appropriate GPA
- See our List of GPAs by IC
-
For Non-NIH funded investigators:
- See How to Submit A Non-NIH funded Study to dbGaP for step-by-step instructions.
Step 2: Send required documents and information to GPA
Prepare and obtain an institutional review board (IRB) review for a signed Institutional Certification, which will be required at Just in Time for NIH funded researchers, before submitting data.
Gather basic study information, which must be later entered in dbGaP during registration. Below is a list of
standard information collected at this stage.
Some funding institutes or centers (ICs) may require additional information, and/or require that the
investigator submit this information to the IC before the registration process. Investigators should
discuss
with their GPA (see Step 1 below) as early as possible to find out whether this is the case.
- Target dates for data submission and data release
- Number of participants
- Data type
- Secondary contact information
- Consent groups
- Acknowledgement statement
Step 3: GPA registers the study in dbGaP
- The GPA will initiate registration in the dbGaP system using the Principal Investigator (PI) name, study name, and grant number (extramural) or protocol number (intramural) when the basic study information has been received and the Institutional Certifications have been approved.
Step 4 (Optional): The Principal Investigator (PI) receives an “invite” from the dbGaP system
Once the GPA has initiated the registration in dbGaP, the PI may receive an email with instructions for completing the study registration process.
Step 5: PI completes the study registration in dbGaP
The PI (or designee) enters basic study information in dbGaP (see Step 2 above), and can update as needed
- The GPA (optionally with the PI) uploads the signed Institutional
Certification and creates a Data Use Certification in dbGaP.
- A Data Use Certification describes the terms and conditions of using the data from the uploaded study.
- The Institutional Certification assures NIH that the study submission complies with the
GDS policy and sets limits, if any, on how other researchers may use the dataset.
-
For Extramural (Grants & Contracts) and NIH Intramural investigators:
- should have completed a signed institutional certification submitted as part of JIT and prior to award
- For Non-NIH funded investigators:
- will need to complete a signed institutional certification to accompany their study submission. Learn more about registering a study in dbGaP for non-NIH funded investigators.
-
For Extramural (Grants & Contracts) and NIH Intramural investigators:
Step 6: GPA verifies the study registration information.
- Once the GPA verifies the study registration in dbGaP, the PI or designee will receive an email invitation to the dbGaP submission portal.
- The registration system changes the study to “Complete” and locks the study from any further changes until it is released.
Step 7: PI submits data to dbGaP and/or another repository
Under the NIH GDS policy, large-scale human genomic studies must be registered in dbGaP, but the data may be submitted to dbGaP and/or another repository, per the approved GDS plan.
Step 7a: If submitting data to dbGaP
- For those investigators submitting data to dbGaP, a detailed study
submission guide is available.
- After submission of the metadata to dbGaP, the PI receives a study accession number and a password-protected preview site to review and approve.
- The dbGaP team will process and prepare the data.
- When the data will be submitted to a repository other than dbGaP, the study will have a public page on dbGaP with the metadata and provide access control when necessary.
- After the PI approves the preview site, dbGaP publishes a public display of summary-level data and controlled-access release of individual-level data.
- Studies that have been granted an alternate data sharing plan may not have to submit data to dbGaP, but must still register the study in dbGaP.
Step 7b: If submitting data to a repository other than dbGaP
- Investigators not submitting data to dbGaP must:
- Register the study in dbGaP
- Submit study metadata to dbGaP